

Great for chemistry students and chemists, these science flash cards feature scientific terms.
There are 18 flash cards in this set (5 pages to print.)
To use:
1. Print out the cards.
2. Cut along the dashed lines.
3. Fold along the solid lines.
Sample flash cards in this set:

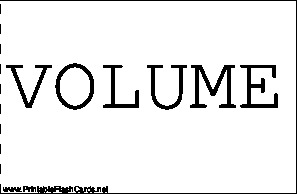


| Questions | Answers |
|---|---|
| MASS | A MEASUREMENT OF THE AMOUNT OF MATTER IN AN OBJECT |
| VOLUME | THE AMOUNT OF SPACE OCCUPIED BY AN OBJECT |
| DENSITY | THE MASS PER UNIT VOLUE OF A MATERIAL/HOW TIGHTLY PACKED A SUBSTANCE'S MOLECULES ARE |
| SCIENTIFIC THEORY | THE MOST LOGICAL EXPLANATION OF WHY THINGS WORK THE WAY THEY DO |
| KINETIC THEORY (OF MATTER) | THE IDEA THAT ALL MATTER IS MADE UP OF CONSTANTLY MOVING, TINY PARTICLES |
| EVAPORATION | THE GRADUAL CHANGE OF A SUBSTANCE FROM A LIQUID TO A GAS AT TEMPERATURES BELOW THE BOILING POINT |
| CONDENSATION | THE CHANGE OF A SUBSTANCE FROM A GAS TO A LIQUID |
| MELTING POINT | THE TEMPERATURE AT WHICH A SOLID CHANGES TO A LIQUIED |
| HEAT OF FUSION | THE AMOUNT OF ENERGY NEEDED TO CHANGE A MATERIAL FROM THE SOLID STATE TO THE LIQUID STATE |
| BOILING POINT | THE TEMPERATURE AT WHICH VAPOR BUBBLES FORM IN A LIQUID AND RISE TO THE SURFACE |
| HEAT OF VAPORIZATION | THE AMOUNT OF ENERGY NEEDED TO CHANGE A MATERIAL FROM A LIQUID TO A GAS |
| PLASMA | A GASLIKE MIXTURE OF CHARGED PARTICLES THAT EXISTS AT EXTREMELY HIGH TEMPERATURES |
| PRESSURE | THE AMOUNT OF FORCE EXERTED PER UNIT OF AREA |
| TEMPERATURE | A MEASURE OF THE AVERAGE KINETIC ENERGY OF THE PARTICLES THAT MAKE UP A SAMPLE OF MATTER |
| SI | INTERNATIONAL SYSTEM OF UNITS |
| THERMAL EXPANSION | A CHARACTERISTIC OF ALMOST ALL MATTER THAT CAUSES IT TO EXPAND WHEN HEATED AND CONTRACT WHEN COOLED |
| ELEMENT | SUBSTANCE IN WHICH ALL THE ATOMS IN A SAMPLE ARE ALIKE |
| CHEMICAL CHANGE | THE CHANGE OF SUBSTANCES TO DIFFERENT SUBSTANCES |