
Chemistry students can learn about elements and periodic chart with these printable science flash cards.
There are 22 flash cards in this set (4 pages to print.)
To use:
1. Print out the cards.
2. Cut along the dashed lines.
3. Fold along the solid lines.
Sample flash cards in this set:




| Questions | Answers |
|---|---|
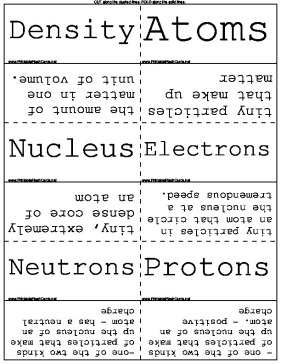
| Density | the amount of matter in one unit of volume. |
| Atoms | tiny particles that make up matter |
| Nucleus | tiny, extremely dense core of an atom |
| Electrons | tiny particles in an atom that circle the nucleus at a tremendous speed. |
| Neutrons | -one of the two kinds of particles that make up the nucleus of an atom - has a neutral charge |
| Protons | - one of the two kinds of particles that make up the nucleus of an atom. - positive charge |
| Neutral | lacking an electrical charge |
| Shell | a set of electrons in an atom that orbit the nucleus at roughly the same distance |
| Atomic Number | the number of protons in an atom |
| Element | a substance that is composed of only one type of atom. |
| Compound | a substance that is composed of more than one type of atom bonded together. |
| Metals | Elements located on - the left and center in the periodic table - most are silvery in color - most are solid at room temperature |
| Nonmetal | - located at the right side of the periodic table. - many solids and several gases |
| Periodic Table of Elements | a chart constructed by Dmitri Mendeleev to arrange the elements in such a way as to group similar elements together. |
| Halogen | - Elements next to the last column of the periodic table. - shows a tendency to form compounds with alkali and alkaline earth metals. |
| Sublime | to turn from a solid into a gas |
| Noble Gases | - elements in the last column of the periodic table - gases - do not combine with other elements except under very unusual circumstances. |
| Molecule | tiny group of two or more atoms that are bonded tightly together. |
| Crystal | a geometric arrangement of atoms |
| Ionic Compound | a compound composed of charged atoms or groups of atoms. |
| Chemical reaction | When atoms of elements or compounds are rearranged to form new substances |
| Combustion | - burning - a chemical reaction in which a substance reacts rapidly with oxygen |